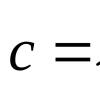Experimental natural science of the 17th century
In the 15th-16th centuries, a period of rapid growth in trade and material production began in Europe. By the 16th century, technology in Europe had reached a level significantly higher than during the heyday of the Ancient World. At the same time, changes in technical techniques were ahead of their theoretical understanding.
The technical inventions of the 16th century and the brilliant successes of navigation (which, by the way, resolved the centuries-long financial crisis associated with the shortage of precious metals) simultaneously posed new problems for science that previously existing science could not resolve.
Further improvement of technology rested on the main contradiction of the era - the contradiction between the relatively high level of technological knowledge achieved by that time and the sharp lag in theoretical natural science.
The seventeenth century in philosophy was also marked by the revival of atomistic ideas. Mathematician (founder of analytical geometry) and philosopher René Descartes, also known as Cartesius, argued that all bodies are composed of corpuscles of various shapes and sizes; the shape of the corpuscles is related to the properties of the substance. At the same time, Descartes believed that corpuscles are divisible and consist of a single matter. Descartes denied Democritus' ideas about indivisible atoms moving in emptiness, not daring to admit the existence of emptiness. Corpuscular ideas, very close to the ancient ideas of Epicurus, were also expressed by the French philosopher Pierre Gassendi. Gassendi called groups of atoms forming compounds molecules (from lat. moles- a bunch).
Gassendi's corpuscular concepts have gained fairly wide recognition among natural scientists.
In the 17th century, new experimental natural science became a tool for resolving the contradiction between a high level of technology and an extremely low level of knowledge about nature. Enormous advances in the 17th century were achieved in the fields of physics, mechanics, mathematics and astronomy. Galileo Galilei not only founded classical mechanics, but also introduced a new way of thinking into physics that made full use of the experimental method. The German astronomer Johannes Kepler in 1609 brought into conformity with astronomical data the heliocentric system, which was proposed in 1543 by Nicolaus Copernicus, and which in its original form contained many inaccuracies. Evangelista Torricelli, Blaise Pascal and Otto von Guericke conducted it in the mid-17th century., the result of which was the formation of a new natural science, based entirely on experimental data. The principle of quantitative measurement in experimental research becomes the basis of natural science. This is reflected in the invention of various measuring instruments - chronometers, thermometers, hydrometers, barometers, scales, etc.
The new natural science also gave rise to new organizational forms - scientific societies and academies of sciences were created. As early as 1560, the Italian naturalist Giovanni Battista della Porta began holding regular meetings in his home, called the Academy of Natural Mysteries. In the 17th century
Officially established academies with corresponding bodies and statutes appeared: the Academy of Naturalists (Leopoldina) in Germany (1652), the Academy of Experience in Florence (1657), the Royal Society (1662) in London, the Paris Academy of Exact Sciences (1663).
One of the consequences of the scientific revolution that occurred in the second half of the 17th century was the creation of a new - scientific - chemistry. Robert Boyle is traditionally considered the creator of scientific chemistry.
Robert Boyle and the emergence of scientific chemistry
Chemistry of antiquity.
Chemistry, the science of the composition of substances and their transformations, begins with man's discovery of the ability of fire to change natural materials. Apparently, people knew how to smelt copper and bronze, burn clay products, and make glass as early as 4000 BC. By the 7th century. BC. Egypt and Mesopotamia became centers for dye production; Gold, silver and other metals were also obtained there in their pure form. From about 1500 to 350 BC. Distillation was used to produce dyes, and metals were smelted from ores by mixing them with charcoal and blowing air through the burning mixture. The very procedures for transforming natural materials were given a mystical meaning.
Greek natural philosophy.
These mythological ideas penetrated into Greece through Thales of Miletus, who raised all the diversity of phenomena and things to a single element - water. However, Greek philosophers were not interested in the methods of obtaining substances and their practical use, but mainly in the essence of the processes occurring in the world. Thus, the ancient Greek philosopher Anaximenes argued that the fundamental principle of the Universe is air: when rarefied, air turns into fire, and as it thickens, it becomes water, then earth and, finally, stone. Heraclitus of Ephesus tried to explain natural phenomena by postulating fire as the primary element.
These ideas were combined in the natural philosophy of Empedocles from Agrigentum, the creator of the theory of the four principles of the universe. In various versions, his theory dominated the minds of people for more than two thousand years. According to Empedocles, all material objects are formed by the combination of eternal and unchanging elements - water, air, earth and fire - under the influence of the cosmic forces of love (attraction) and hatred (repulsion). Empedocles' theory of elements was accepted and developed first by Plato, who specified that the immaterial forces of good and evil can transform these elements into one another, and then by Aristotle.
According to Aristotle, elemental elements are not material substances, but carriers of certain qualities - heat, cold, dryness and humidity. This view was transformed into Galen’s idea of the four “juices” and dominated science until the 17th century. Another important question that occupied Greek natural philosophers was the question of the divisibility of matter. The founders of the concept, which later received the name “atomistic,” were Leucippus, his student Democritus and Epicurus. According to their teaching, there are only emptiness and atoms - indivisible material elements, eternal, indestructible, impenetrable, differing in shape, position in emptiness and size; from their “vortex” all bodies are formed. Atomistic theory remained unpopular for two millennia after Democritus, but did not disappear completely. One of its adherents was the ancient Greek poet Titus Lucretius Carus, who outlined the views of Democritus and Epicurus in the poem About the nature of things (De Rerum Natura).
Alchemy.
Alchemy is the art of improving matter through the transformation of metals into gold and improving man by creating the elixir of life. Striving to achieve the most attractive goal for them - the creation of incalculable wealth - alchemists solved many practical problems, discovered many new processes, observed various reactions, contributing to the formation of a new science - chemistry.
Hellenistic period.
Egypt was the cradle of alchemy. The Egyptians were brilliant in applied chemistry, which, however, was not isolated as an independent field of knowledge, but was part of the “sacred secret art” of the priests. Alchemy appeared as a separate field of knowledge at the turn of the 2nd and 3rd centuries. AD After the death of Alexander the Great, his empire collapsed, but the influence of the Greeks extended to vast territories of the Near and Middle East. Alchemy reached a particularly rapid flowering in 100–300 AD. in Alexandria.
Around 300 AD. The Egyptian Zosima wrote an encyclopedia - 28 books covering all knowledge of alchemy over the previous 5-6 centuries, in particular information about the interconversions (transmutations) of substances.
Alchemy in the Arab world.
Having conquered Egypt in the 7th century, the Arabs adopted Greco-Oriental culture, preserved for centuries by the Alexandrian school. Imitating the ancient rulers, the caliphs began to patronize the sciences, and in the 7th–9th centuries. the first chemists appeared.
The most talented and famous Arab alchemist was Jabir ibn Hayyan (late 8th century), who later became known in Europe under the name Geber. Jabir believed that sulfur and mercury are two opposite principles from which the seven other metals are formed; Gold is the most difficult to form: for this you need a special substance, which the Greeks called xerion - “dry”, and the Arabs changed to al-iksir (this is how the word “elixir” appeared). The elixir was supposed to have other wonderful properties: to cure all diseases and give immortality. Another Arab alchemist, al-Razi (c. 865–925) (known in Europe as Rhazes) also practiced medicine. Thus, he described the method of preparing plaster and the method of applying a bandage to the fracture site. However, the most famous doctor was the Bukharan Ibn Sina, also known as Avicenna. His writings served as a guide for doctors for many centuries.
Alchemy in Western Europe.
The scientific views of the Arabs penetrated into medieval Europe in the 12th century. through North Africa, Sicily and Spain. The works of Arab alchemists were translated into Latin and then into other European languages. At first, alchemy in Europe relied on the work of such luminaries as Jabir, but three centuries later there was a renewed interest in the teachings of Aristotle, especially in the works of the German philosopher and Dominican theologian, who later became a bishop and professor at the University of Paris, Albertus Magnus and his student Thomas Aquinas. Convinced of the compatibility of Greek and Arabic science with Christian doctrine, Albertus Magnus promoted their introduction into scholastic courses of study. In 1250, Aristotle's philosophy was introduced into teaching at the University of Paris. The English philosopher and naturalist, Franciscan monk Roger Bacon, who anticipated many later discoveries, was also interested in alchemical problems; he studied the properties of saltpeter and many other substances, and found a method for making black gunpowder. Other European alchemists include Arnaldo da Villanova (1235–1313), Raymond Lull (1235–1313), and Basil Valentinus (15th–16th century German monk).
Achievements of alchemy.
The development of crafts and trade, the rise of cities in Western Europe in the 12th–13th centuries. accompanied by the development of science and the emergence of industry. Alchemist recipes were used in technological processes such as metal processing. During these years, a systematic search for ways to obtain and identify new substances began. Recipes for producing alcohol and improving the distillation process are emerging. The most important achievement was the discovery of strong acids - sulfuric and nitric. Now European chemists were able to carry out many new reactions and obtain substances such as salts of nitric acid, vitriol, alum, salts of sulfuric and hydrochloric acids. The services of alchemists, who were often skilled doctors, were used by the highest nobility. It was also believed that alchemists possessed the secret of transmuting ordinary metals into gold.

By the end of the 14th century. The alchemists' interest in transforming certain substances into others gave way to an interest in the production of copper, brass, vinegar, olive oil and various medicines. In the 15th–16th centuries. The experience of alchemists was increasingly used in mining and medicine.
THE BEGINNING OF MODERN CHEMISTRY
The end of the Middle Ages was marked by a gradual retreat from the occult, a decline in interest in alchemy and the spread of a mechanistic view of the structure of nature.
Iatrochemistry.
Paracelsus (1493–1541) held completely different views on the purposes of alchemy. Under this name chosen by himself (“superior to Celsus”), the Swiss physician Philip von Hohenheim entered history. Paracelsus, like Avicenna, believed that the main task of alchemy was not the search for ways to obtain gold, but the production of medicines. He borrowed from the alchemical tradition the doctrine that there are three main parts of matter - mercury, sulfur, salt, which correspond to the properties of volatility, flammability and hardness. These three elements form the basis of the macrocosm (Universe) and are associated with the microcosm (man), formed by spirit, soul and body. Moving on to determining the causes of diseases, Paracelsus argued that fever and plague occur from an excess of sulfur in the body, with an excess of mercury paralysis occurs, etc. The principle that all iatrochemists adhered to was that medicine is a matter of chemistry, and everything depends on the ability of the doctor to isolate pure principles from impure substances. Within this scheme, all body functions were reduced to chemical processes, and the alchemist's task was to find and prepare chemical substances for medical purposes.
The main representatives of the iatrochemical direction were Jan Helmont (1577–1644), a doctor by profession; Francis Sylvius (1614–1672), who enjoyed great fame as a physician and eliminated “spiritual” principles from iatrochemical teaching; Andreas Liebavius (c. 1550–1616), physician from Rothenburg. Their research greatly contributed to the formation of chemistry as an independent science.
Mechanistic philosophy.
With the decrease in the influence of iatrochemistry, natural philosophers again turned to the teachings of the ancients about nature. To the fore in the 17th century. atomistic (corpuscular) views emerged. One of the most prominent scientists - the authors of the corpuscular theory - was the philosopher and mathematician Rene Descartes. He outlined his views in 1637 in the essay Reasoning about the method. Descartes believed that all bodies “consist of numerous small particles of various shapes and sizes, ... which do not fit each other so exactly that there are no gaps around them; these gaps are not empty, but filled with... rarefied matter.” Descartes did not consider his “little particles” to be atoms, i.e. indivisible; he stood on the point of view of the infinite divisibility of matter and denied the existence of emptiness. One of Descartes' most prominent opponents was the French physicist and philosopher Pierre Gassendi. Gassendi's atomism was essentially a retelling of the teachings of Epicurus, however, unlike the latter, Gassendi recognized the creation of atoms by God; he believed that God created a certain number of indivisible and impenetrable atoms, of which all bodies are composed; There must be absolute emptiness between the atoms. In the development of chemistry in the 17th century. a special role belongs to the Irish scientist Robert Boyle. Boyle did not accept the statements of ancient philosophers who believed that the elements of the universe could be established speculatively; this is reflected in the title of his book Skeptical chemist. Being a supporter of the experimental approach to determining chemical elements (which was ultimately adopted), he did not know about the existence of real elements, although he almost discovered one of them - phosphorus - himself. Boyle is usually credited with introducing the term "analysis" into chemistry. In his experiments on qualitative analysis, he used various indicators and introduced the concept of chemical affinity. Based on the works of Galileo Galilei Evangelista Torricelli, as well as Otto Guericke, who demonstrated the “Magdeburg hemispheres” in 1654, Boyle described the air pump he designed and experiments to determine the elasticity of air using a U-shaped tube. As a result of these experiments, the well-known law of inverse proportionality between air volume and pressure was formulated. In 1668, Boyle became an active member of the newly organized Royal Society of London, and in 1680 he was elected its president.
Technical chemistry.
Scientific advances and discoveries could not but influence technical chemistry, elements of which can be found in the 15th–17th centuries. In the middle of the 15th century. blower forge technology was developed. The needs of the military industry stimulated work to improve the technology of gunpowder production. During the 16th century. Gold production doubled and silver production increased ninefold. Fundamental works are being published on the production of metals and various materials used in construction, glass making, fabric dyeing, food preservation, and leather tanning. With the expansion of consumption of alcoholic beverages, distillation methods are being improved and new distillation apparatuses are being designed. Numerous production laboratories, primarily metallurgical ones, appeared. Among the chemical technologists of that time we can mention Vannoccio Biringuccio (1480–1539), whose classic work ABOUT pyrotechnics was printed in Venice in 1540 and contained 10 books that dealt with mines, testing minerals, preparing metals, distillation, the art of war and fireworks. Another famous treatise About mining and metallurgy, was written by Georg Agricola (1494–1555). Mention should also be made of Johann Glauber (1604–1670), a Dutch chemist who created Glauber's salt.
EIGHTEENTH CENTURY
Chemistry as a scientific discipline.
From 1670 to 1800, chemistry received official status in the curricula of leading universities, along with natural philosophy and medicine. In 1675 the textbook of Nicolas Lemery (1645–1715) appeared Chemistry course, which gained enormous popularity, 13 of its French editions were published, and in addition, it was translated into Latin and many other European languages. In the 18th century scientific chemical societies and a large number of scientific institutes are being created in Europe; The research they conduct is closely related to the social and economic needs of society. Practicing chemists appeared, engaged in the manufacture of instruments and the production of substances for industry.
Phlogiston theory.
In the works of chemists of the second half of the 17th century. Much attention was paid to interpretations of the combustion process. According to the ancient Greeks, everything that can burn contains the element of fire, which is released under the right conditions. In 1669, the German chemist Johann Joachim Becher tried to give a rationalistic explanation of flammability. He suggested that solids consist of three types of “earth,” and one of the types, which he called “greasy earth,” was taken to be the “principle of flammability.”
Becher's follower, the German chemist and physician Georg Ernst Stahl, transformed the concept of “fat earth” into the generalized doctrine of phlogiston - “the beginning of flammability.” According to Stahl, phlogiston is a certain substance contained in all combustible substances and released during combustion. Stahl argued that the rusting of metals is similar to the burning of wood. Metals contain phlogiston, but rust (scale) no longer contains phlogiston. This also provided an acceptable explanation for the process of converting ores into metals: ore, the content of phlogiston in which is insignificant, is heated on charcoal rich in phlogiston, and the latter turns into ore. Coal turns into ash, and ore into metal rich in phlogiston. By 1780, the phlogiston theory was accepted by chemists almost everywhere, although it did not answer a very important question: why does iron become heavier when it rusts, although phlogiston evaporates from it? Chemists of the 18th century this contradiction did not seem so important; the main thing, in their opinion, was to explain the reasons for the change in the appearance of substances.
In the 18th century There were many chemists whose scientific activities do not fit into the usual schemes for considering the stages and directions of development of science, and among them a special place belongs to the Russian encyclopedist scientist, poet, and champion of enlightenment Mikhail Vasilyevich Lomonosov (1711–1765). With his discoveries, Lomonosov enriched almost all areas of knowledge, and many of his ideas were more than a hundred years ahead of the science of that time. In 1756, Lomonosov conducted famous experiments on burning metals in a closed vessel, which provided indisputable evidence of the preservation of matter during chemical reactions and the role of air in combustion processes: even before Lavoisier, he explained the observed increase in weight when burning metals by combining them with air. In contrast to the prevailing ideas about caloric, he argued that thermal phenomena are caused by the mechanical movement of material particles. He explained the elasticity of gases by the movement of particles. Lomonosov distinguished between the concepts of “corpuscle” (molecule) and “element” (atom), which received general recognition only in the mid-19th century. Lomonosov formulated the principle of conservation of matter and motion, excluded phlogiston from the list of chemical agents, laid the foundations of physical chemistry, and created a chemical laboratory at the St. Petersburg Academy of Sciences in 1748, in which not only scientific work was carried out, but also practical classes for students. He conducted extensive research in areas of knowledge related to chemistry - physics, geology, etc.
Pneumatic chemistry.
The shortcomings of the phlogiston theory most clearly emerged during the development of the so-called. pneumatic chemistry. The largest representative of this trend was R. Boyle: he not only discovered the gas law, which now bears his name, but also designed devices for collecting air. Chemists now have a vital means of isolating, identifying, and studying various “airs.” An important step was the invention of the “pneumatic bath” by the English chemist Stephen Hales (1677–1761) in the early 18th century. - a device for trapping gases released when a substance is heated into a vessel of water, lowered upside down into a bath of water. Later, Hales and Henry Cavendish established the existence of certain gases (“airs”) that differ in their properties from ordinary air. In 1766, Cavendish systematically studied the gas formed by the reaction of acids with certain metals, later called hydrogen. A great contribution to the study of gases was made by the Scottish chemist Joseph Black. He began to study the gases released when acids react with alkalis. Black discovered that the mineral calcium carbonate decomposes when heated, releasing gas and forming lime (calcium oxide). The released gas (carbon dioxide - Black called it "bound air") could be recombined with lime to form calcium carbonate. Among other things, this discovery established the inseparability of bonds between solid and gaseous substances.
Chemical revolution.
Joseph Priestley, a Protestant priest who was passionate about chemistry, achieved great success in isolating gases and studying their properties. Near Leeds (England), where he served, there was a brewery from which large quantities of “bound air” (we now know that it was carbon dioxide) could be obtained for experiments. Priestley discovered that gases could be dissolved in water, and tried to collect them not over water, but over mercury. So he was able to collect and study nitric oxide, ammonia, hydrogen chloride, sulfur dioxide (of course, these are their modern names). In 1774, Priestley made his most important discovery: he isolated a gas in which substances burned especially brightly. Being a proponent of the phlogiston theory, he called this gas “dephlogisticated air.” The gas discovered by Priestley seemed to be the antithesis of “phlogisticated air” (nitrogen), isolated in 1772 by the English chemist Daniel Rutherford (1749–1819). In “phlogisticated air” the mice died, but in “dephlogisticated” air they were very active. (It should be noted that the properties of the gas isolated by Priestley were described by the Swedish chemist Karl Wilhelm Scheele back in 1771, but his message, due to the negligence of the publisher, appeared in print only in 1777.) The great French chemist Antoine Laurent Lavoisier immediately appreciated the significance of Priestley’s discovery. In 1775, he prepared an article in which he argued that air is not a simple substance, but a mixture of two gases, one of them is Priestley’s “dephlogisticated air,” which combines with burning or rusting objects, passes from ores to charcoal and is necessary for life. Lavoisier called him oxygen, oxygen, i.e. "acid-generating" The second blow to the theory of elemental elements was dealt after it became clear that water is also not a simple substance, but a product of a combination of two gases: oxygen and hydrogen. All these discoveries and theories, having done away with the mysterious “elements,” led to the rationalization of chemistry. Only those substances that can be weighed or the amount of which can be measured in some other way have come to the fore. During the 80s of the 18th century. Lavoisier, in collaboration with other French chemists Antoine François de Fourcroy (1755–1809), Guiton de Morveau (1737–1816) and Claude Louis Berthollet, developed a logical system of chemical nomenclature; it described more than 30 simple substances indicating their properties. This work Chemical nomenclature method, was published in 1787.
A revolution in the theoretical views of chemists that occurred at the end of the 18th century. as a result of the rapid accumulation of experimental material under the dominance of the phlogiston theory (albeit independently of it), it is usually called the “chemical revolution”.
NINETEENTH CENTURY
Composition of substances and their classification.
Lavoisier's successes showed that the use of quantitative methods can help in determining the chemical composition of substances and elucidating the laws of their association.
Atomic theory.
The birth of physical chemistry.
By the end of the 19th century. The first works appeared in which the physical properties of various substances (boiling and melting points, solubility, molecular weight) were systematically studied. Such research was started by Gay-Lussac and Van't Hoff, who showed that the solubility of salts depends on temperature and pressure. In 1867, Norwegian chemists Peter Waage (1833–1900) and Kato Maximilian Guldberg (1836–1902) formulated the law of mass action, according to which the rate of reactions depends on the concentrations of the reactants. The mathematical apparatus they used made it possible to find a very important quantity that characterizes any chemical reaction - the rate constant.
Chemical thermodynamics.
Meanwhile, chemists turned to the central question of physical chemistry - the influence of heat on chemical reactions. By the middle of the 19th century. physicists William Thomson (Lord Kelvin), Ludwig Boltzmann and James Maxwell developed new views on the nature of heat. Rejecting Lavoisier's caloristic theory, they represented heat as the result of movement. Their ideas were developed by Rudolf Clausius. He developed a kinetic theory according to which quantities such as volume, pressure, temperature, viscosity and reaction rates can be considered based on the idea of the continuous movement of molecules and their collisions. Simultaneously with Thomson (1850), Clasius gave the first formulation of the second law of thermodynamics and introduced the concepts of entropy (1865), ideal gas, and the mean free path of molecules.
The thermodynamic approach to chemical reactions was used in his works by August Friedrich Gorstmann (1842–1929), who, based on the ideas of Clausius, tried to explain the dissociation of salts in solution. In 1874–1878, the American chemist Josiah Willard Gibbs undertook a systematic study of the thermodynamics of chemical reactions. He introduced the concept of free energy and chemical potential, explaining the essence of the law of mass action, and applied thermodynamic principles in studying the equilibrium between different phases at different temperatures, pressures and concentrations (phase rule). Gibbs' work laid the foundation for modern chemical thermodynamics. Swedish chemist Svante August Arrhenius created the theory of ionic dissociation, which explains many electrochemical phenomena, and introduced the concept of activation energy. He also developed an electrochemical method for measuring the molecular weight of solutes.
A major scientist, thanks to whom physical chemistry was recognized as an independent field of knowledge, was the German chemist Wilhelm Ostwald, who applied Gibbs' concepts in the study of catalysis. In 1886 he wrote the first textbook on physical chemistry, and in 1887 he founded (together with Van't Hoff) the journal Physical Chemistry (Zeitschrift für physikalische Chemie).
THE TWENTIETH CENTURY
New structural theory.
With the development of physical theories about the structure of atoms and molecules, such old concepts as chemical affinity and transmutation were rethought. New ideas about the structure of matter emerged.
Atom model.
In 1896, Antoine Henri Becquerel (1852–1908) discovered the phenomenon of radioactivity, discovering the spontaneous emission of subatomic particles from uranium salts, and two years later, the spouses Pierre Curie and Marie Sklodowska-Curie isolated two radioactive elements: polonium and radium. In subsequent years, it was discovered that radioactive substances emit three types of radiation: a-particles, b-particles and g-rays. Together with the discovery of Frederick Soddy, which showed that during radioactive decay the transformation of some substances into others occurs, all this gave new meaning to what the ancients called transmutation.
In 1897, Joseph John Thomson discovered the electron, the charge of which was measured with high accuracy in 1909 by Robert Millikan. In 1911, Ernst Rutherford, based on Thomson's electron concept, proposed a model of the atom: at the center of the atom there is a positively charged nucleus, and negatively charged electrons revolve around it. In 1913, Niels Bohr, using the principles of quantum mechanics, showed that electrons can be located not in any, but in strictly defined orbits. The Rutherford-Bohr planetary quantum model of the atom forced scientists to take a new approach to explaining the structure and properties of chemical compounds. German physicist Walter Kossel (1888–1956) suggested that the chemical properties of an atom are determined by the number of electrons in its outer shell, and the formation of chemical bonds is determined mainly by the forces of electrostatic interaction. American scientists Gilbert Newton Lewis and Irving Langmuir formulated the electronic theory of chemical bonding. In accordance with these ideas, molecules of inorganic salts are stabilized by electrostatic interactions between their constituent ions, which are formed during the transfer of electrons from one element to another (ionic bond), and molecules of organic compounds - due to the sharing of electrons (covalent bond). These ideas underlie modern concepts of chemical bonding.
New research methods.
All new ideas about the structure of matter could only be formed as a result of the development in the 20th century. experimental techniques and the emergence of new research methods. The discovery of X-rays in 1895 by Wilhelm Conrad Roentgen served as the basis for the subsequent creation of the method of X-ray crystallography, which makes it possible to determine the structure of molecules from the diffraction pattern of X-rays on crystals. Using this method, the structure of complex organic compounds was deciphered - insulin, deoxyribonucleic acid (DNA), hemoglobin, etc. With the creation of atomic theory, new powerful spectroscopic methods appeared that provide information about the structure of atoms and molecules. Various biological processes, as well as the mechanism of chemical reactions, are studied using radioisotope tracers; Radiation methods are also widely used in medicine.
Biochemistry.
This scientific discipline, which studies the chemical properties of biological substances, was first one of the branches of organic chemistry. It became an independent region in the last decade of the 19th century. as a result of studies of the chemical properties of substances of plant and animal origin. One of the first biochemists was the German scientist Emil Fischer. He synthesized substances such as caffeine, phenobarbital, glucose, and many hydrocarbons, and made a great contribution to the science of enzymes - protein catalysts, first isolated in 1878. The formation of biochemistry as a science was facilitated by the creation of new analytical methods. In 1923, Swedish chemist Theodor Svedberg designed an ultracentrifuge and developed a sedimentation method for determining the molecular weight of macromolecules, mainly proteins. Svedberg's assistant Arne Tiselius (1902–1971) in the same year created the method of electrophoresis, a more advanced method for separating giant molecules based on the difference in the speed of migration of charged molecules in an electric field. At the beginning of the 20th century. Russian chemist Mikhail Semenovich Tsvet (1872–1919) described a method for separating plant pigments by passing their mixture through a tube filled with an adsorbent. The method was called chromatography. In 1944, English chemists Archer Martin and Richard Singh proposed a new version of the method: they replaced the tube with the adsorbent with filter paper. This is how paper chromatography appeared - one of the most common analytical methods in chemistry, biology and medicine, with the help of which in the late 1940s and early 1950s it was possible to analyze mixtures of amino acids resulting from the breakdown of different proteins and determine the composition of proteins. As a result of painstaking research, the order of amino acids in the insulin molecule was established (Frederick Sanger), and by 1964 this protein was synthesized. Nowadays, many hormones, medicines, and vitamins are obtained using biochemical synthesis methods.

Industrial chemistry.
Probably the most important stage in the development of modern chemistry was the creation in the 19th century. various research centers engaged in, in addition to fundamental, also applied research. At the beginning of the 20th century. a number of industrial corporations created the first industrial research laboratories. In the USA, the DuPont chemical laboratory was founded in 1903, and the Bell laboratory was founded in 1925. After the discovery and synthesis of penicillin in the 1940s, and then other antibiotics, large pharmaceutical companies emerged, staffed by professional chemists. Work in the field of chemistry of macromolecular compounds was of great practical importance. One of its founders was the German chemist Hermann Staudinger (1881–1965), who developed the theory of the structure of polymers. Intensive searches for methods for producing linear polymers led in 1953 to the synthesis of polyethylene (Karl Ziegler), and then other polymers with desired properties. Today, polymer production is the largest branch of the chemical industry.
Not all advances in chemistry have been beneficial to humans. In the 19th century In the production of paints, soap, and textiles, hydrochloric acid and sulfur were used, which posed a great danger to the environment. In the 20th century The production of many organic and inorganic materials has increased due to the recycling of used substances, as well as through the processing of chemical wastes that pose a risk to human health and the environment.
Literature:
Figurovsky N.A. Essay on the general history of chemistry. M., 1969
Jua M. History of chemistry. M., 1975
Azimov A. A Brief History of Chemistry. M., 1983
Joseph Priestley, a Protestant priest who was passionate about chemistry, achieved great success in isolating gases and studying their properties. Near Leeds (England), where he served, there was a brewery from which large quantities of “bound air” (we now know that it was carbon dioxide) could be obtained for experiments. Priestley discovered that gases could be dissolved in water, and tried to collect them not over water, but over mercury. So he was able to collect and study nitric oxide, ammonia, hydrogen chloride, sulfur dioxide (of course, these are their modern names). In 1774, Priestley made his most important discovery: he isolated a gas in which substances burned especially brightly. Being a proponent of the phlogiston theory, he called this gas “dephlogisticated air.” The gas discovered by Priestley seemed to be the antipode of “phlogisticated air” (nitrogen), isolated in 1772 by the English chemist Daniel Rutherford (1749-1819). In “phlogisticated air” the mice died, but in “dephlogisticated” air they were very active. (It should be noted that the properties of the gas isolated by Priestley were described by the Swedish chemist Karl Wilhelm Scheele back in 1771, but his message, due to the negligence of the publisher, appeared in print only in 1777.) The great French chemist Antoine Laurent Lavoisier immediately appreciated the significance of Priestley’s discovery. In 1775, he prepared an article in which he argued that air is not a simple substance, but a mixture of two gases, one of them is Priestley’s “dephlogisticated air,” which combines with burning or rusting objects, passes from ores to charcoal and is necessary for life. Lavoisier called it oxygen, oxygen, i.e. "acid-generating" The second blow to the theory of elemental elements was dealt after it became clear that water is also not a simple substance, but a product of a combination of two gases: oxygen and hydrogen. All these discoveries and theories, having done away with the mysterious “elements,” led to the rationalization of chemistry. Only those substances that can be weighed or the amount of which can be measured in some other way have come to the fore. During the 80s of the 18th century. Lavoisier, in collaboration with other French chemists - Antoine François de Fourcroy (1755-1809), Guiton de Morveau (1737-1816) and Claude Louis Berthollet - developed a logical system of chemical nomenclature; it described more than 30 simple substances indicating their properties. This work, Method of Chemical Nomenclature, was published in 1787.
A revolution in the theoretical views of chemists that occurred at the end of the 18th century. as a result of the rapid accumulation of experimental material under the dominance of the phlogiston theory (albeit independently of it), it is usually called the “chemical revolution”.
Chemistry information
Willstatter, Richard
German chemist Richard Martin Willstätter was born in Karlsruhe, the son of a textile merchant, Max Willstätter and Sophia (Ullmann) Willstätter. He graduated from school in Karlsruhe and the real gymnasium in Nuremberg, where he showed himself so capable...
Tiselius, Arne Wilhelm Kaurin
Swedish biochemist Arne Wilhelm Kaurin Tiselius (Tiselius) was born in Stockholm, the son of Hans Abraham Jason Tiselius, an insurance company employee, and the daughter of a Norwegian priest, Rose (Kaurin) Tiselius. When in 1906 father...
Pt - Platinum
PLATINUM (lat. Platinum), Pt, a chemical element of group VIII of the periodic system, atomic number 78, atomic weight 195.08, belongs to the platinum metals. Properties: density 21.45 g/cm3, melting point 1769 °C. Name: from Spanish...
Since humanity appeared on this planet, it has led a relatively calm and stable lifestyle, consuming the same foods, drawing water from the same sources and breathing the same air. Until recently, there was a fragile balance between us and the rest of nature, and with any kind of environmental or climate change, the balance of power was equalized again thanks to the non-stop course of evolution.
Due to the presence of mental abilities and a certain amount of endurance in our bodies, humans, as a biological species, have developed the ability to intervene in nature and change the environment. The creation of tools, the discovery of fire, the domestication of animals, the cultivation of wild plants, the formation of the first settlements - all these were the first steps on the path to progress and civilization.
This was important for people, but all these were weak attempts, because a person could not cause great harm, since the small population of people was still entirely dependent on the forces of nature and trembled at its slightest whims. Over time, the increasing concentration of people, their invasions became not only more persistent, but also more constant, the nature of these invasions became even more targeted. This led to the fact that, in the end, in the second half of the last century, people's ability to accelerate processes changed so much that “the speed of our own development” began to threaten us.
The brainchild of the Wachowski brothers comes to mind - The Matrix, where, ironically, machines created by people began to use people themselves as biologically beneficial fuel. The current reality prompts thoughts that are so colorfully depicted in the aforementioned blockbuster: people have long been sophisticated in inventing many mechanisms, machines and substances, justifying all this with the desire to “improve” their own lives, that is, to become civilized.

The movie "The Matrix" comes to mind
For greater clarity, let us turn to the history of chemical “inventions” and, as already said, look at the second half of the last century in numbers. The graph clearly shows the increase in the number of inventions of chemical substances in the second half of the twentieth century. As you can see, in the 50s of the last century a real boom in the chemical industry began, and by 1975 statistics recorded 1,000,000 synthetic chemical materials. Further “successes” of chemists in various countries were characterized by the addition of about 1000 new chemicals annually. By the end of the last millennium, humanity was “in use”, i.e. There were more than 60,000 artificially produced chemicals in widespread use.

Graph showing the growth in the number of chemicals over the years of the last century
The largest number of “inventions” of this kind concern the weakest links in the life support chain of humanity, namely:
production of commonly used materials
* insulators
* coverings
production and consumption of the most commonly consumed products
* nutritional supplements
* substances used in processing and storage
*substances used in medicines
use of common and accessible energy sources and media
* air

A wide variety of chemicals have become part of our lives.
This cycle of chemicals we have created is already part of our lives; and we, like any species, must use it, adapt to it, or, least of all, avoid it in order to survive. This concept can be understood if we accept the fact of our own participation, yes, participation, in this continuous process - on the one hand, we are producers, and on the other hand, we are a product of this cycle. Therefore, any turn in our own development or our knowledge turns on ourselves.
At times, our experiments benefited us, as was the case with penicillin, which saved more than one million lives in wars and in peacetime. And there are those that even their discoverers themselves would like to forget about - it is appropriate to recall one of the most powerful weapons of mass destruction, Sarin gas (which was discovered by fateful accident by German chemists who were trying to make pesticides more effective, just on the eve of World War II) . The nature of the third discoveries is not clear to us, as well as our own, since they simply change ourselves: there is probably no need to give examples of the influence of narcotic drugs on the human body. Although at the dawn of pharmacy in the Old World, and then in other parts of the world, they were presented as medicines that people needed.
It would seem that if some substance was invented with the benefit of people in mind, then why do some facts emerge that we did not even suspect existed? In practice, everything is quite simple - the danger of artificial substances lies precisely in the fact that we do not know anything with any reliable accuracy about their effect on what they come into contact with throughout their uncontrolled existence.
This can be shown with an elementary example: we have long known, as it seems to us, everything about oxygen. Oxygen is extremely critical to our body, but pure oxygen can kill us. Since oxygen is not found in nature without impurities, we are not able to consume it in this form. As you can see, we participate in the chains of life exactly as Nature has taught us; and any deviation (and here we tried to improve the substance we need) turns out to be fatal. There is only one conclusion here: what we can be absolutely sure of with any substance is that we do not know how long its potentially harmful effects may not manifest themselves.
One of the essential attributes of the revolution, which we also observe today with increasing alarm, is the unspoken ban on freedom of information regarding invented products, ingredients, compositions and their labeling. Although more and more countries are introducing mandatory requirements for providing information on the composition of food, medicine, clothing, etc., it is still almost impossible in everyday life to determine what, for example, your washing powder, paint, plastic product, etc. consists of. anything! The most provocative thing in this regard is the concealment of persons who are directly involved in the establishment of this secrecy regime.
The excess of unnecessary chemicals has already become so obvious that no one is excited about the invention of a new material, polymer or substitute. The main confirmation of this is the growing desire of people for environmentally friendly products. “The road to hell is paved with good intentions,” one could say about the path that all people need to go through in order to prevent the “victory of the chemical revolution.”
Recent trends in scientific advances indicate a greater shift towards biology, genetics and all things green. Most likely, people will have their eyes “opened” to the endless possibilities of nature beyond chemistry and nuclear energy, and they will come to the conclusion that if the supply of something is not renewable, then there is probably no point in making long-term plans for this finite element.
If you liked this material, then we offer you a selection of the best materials on our site according to our readers. You can find a selection of TOP materials about the new person, the new economy, outlook on the future and education where it is most convenient for youwork. Now he collaborated with the famous physicist and mathematician Pierre Simon Laplace. They managed to construct a special apparatus with which it was possible to measure the heat released as a result of the combustion of substances. It was the so-called ice calorimeter. The researchers also conducted a detailed study of the heat that living organisms emit. By measuring the amount of carbon dioxide exhaled and the heat generated by the body, they proved that food “burns” in the body in a special way. The heat generated by this combustion serves to maintain normal body temperature.
The Lavoisier-Laplace ice calorimeter made it possible back in the 18th century to measure the heat capacities of many solids and liquids, as well as the heat of combustion of various fuels and the heat released by living organisms. For example, the heat given off by an animal (or other object) in the inner chamber was spent on melting the ice in the inner “ice jacket”. The external one served to maintain the temperature of the internal part constant. The heat released was measured by weighing the melt water that flowed into the vessel.